Give Names for the Anion in the Ionic Compounds Below.
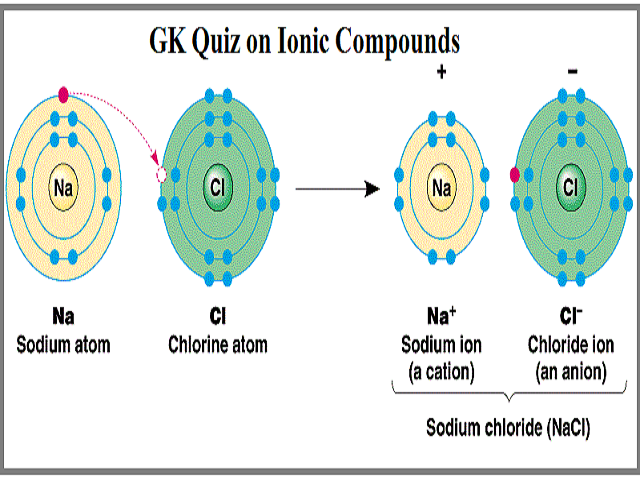
The cation has the same name as its element. Ionic compounds contain a combination of oppositely charged cations anions in a specific ratio to produce a compound with a given whole-number.
Chemistry questions and answers.

. Neutral atom - a chemical species with equal numbers of protons and electrons. Give the formula of the ionic compound magnesium phosphide. Name Cation Anion Compound Formula sodium iodide Na I NaI calcium bromide Ca 2 Br CaBr 2 lithium oxide Li O 2 Li 2 O aluminum sulfide Al 3 S 2 Al 2 S 3 potassium nitride K N 3 K 3 N magnesium nitrate Mg 2 NO 3 MgNO 3 2 sodium sulfate Na SO 4 2 Na 2 SO 4 ammonium phosphate NH 4 PO 4 3 NH 4 3 PO 4 Binary Molecular Compounds If the.
For example the salt ammonium sulfate consists of the cation NH 4 and the sulfate anion SO 42-. - Name the cation if necessary using a Roman numeral to indicate its charge. EqNaCl eq Sodium Chloride.
Chlorine typically forms an ion with a charge of -1. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl. In the name of an ionic compound the cation is listed first and the anion second.
Tellurium gain 2 anion e. The names are found by finding the intersection between the cations and anions. Write the name of each binary ionic compound given below.
As shown before the ions of magnesium and phosphorus are Mg 2 and P 3. - Determine the charge on the cation. Anionsr usually have an ide suffix.
Bromine gain 1 anion. Bas so 07 9 8. C sodium II sulfide.
When the cation andor the anion is a polyatomic ion parentheses may be used to group the atoms in the ion together to write the formula. Chemistry questions and answers. - Write the name of the ionic compound by writing the name of the cation first then the anion.
Order the steps involved in writing a formula for an ionic compound if given the names of its component ions. Anion - a chemical species with more electrons than protons. From a list of almost 2000 names and formulas students will be given the opportunity to practice their ability to name ionic compounds given the formula and determine the formula given the name.
Phosphorus gain 3 anion. Cl- -- Chloride NaCl -- Sodium Chloride Li3N -- Lithium Nitride Directions. The anion is named by taking the elemental name removing the ending and adding ide.
Select the name of Na 2 CO 3. Write the name of the ionic compound by writing the name of the cation first then the anion. For example K 1 is called the potassium ion just as K is called the potassium atom.
Name the cation if necessary using a Roman numeral to indicate its charge. The Stock Method of Naming. The formula of the salt is written as NH 4 2 SO 4.
Given the name select the correct formula for each ionic compound. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. An ionic compound is named first by its cation and then by its anion.
A Mg Cl042 b NH4NO3 c Cu CH3CO22 d K2S03. If needed use the systematic names. Give the name and symbol of the ion formed when.
Below is a chemistry quiz on ionic compounds names and formulas give it a shot and see if you understood all we covered in this topic on Ions. Aluminum lose 3 cation d. Cation - a chemical species with protons than electrons.
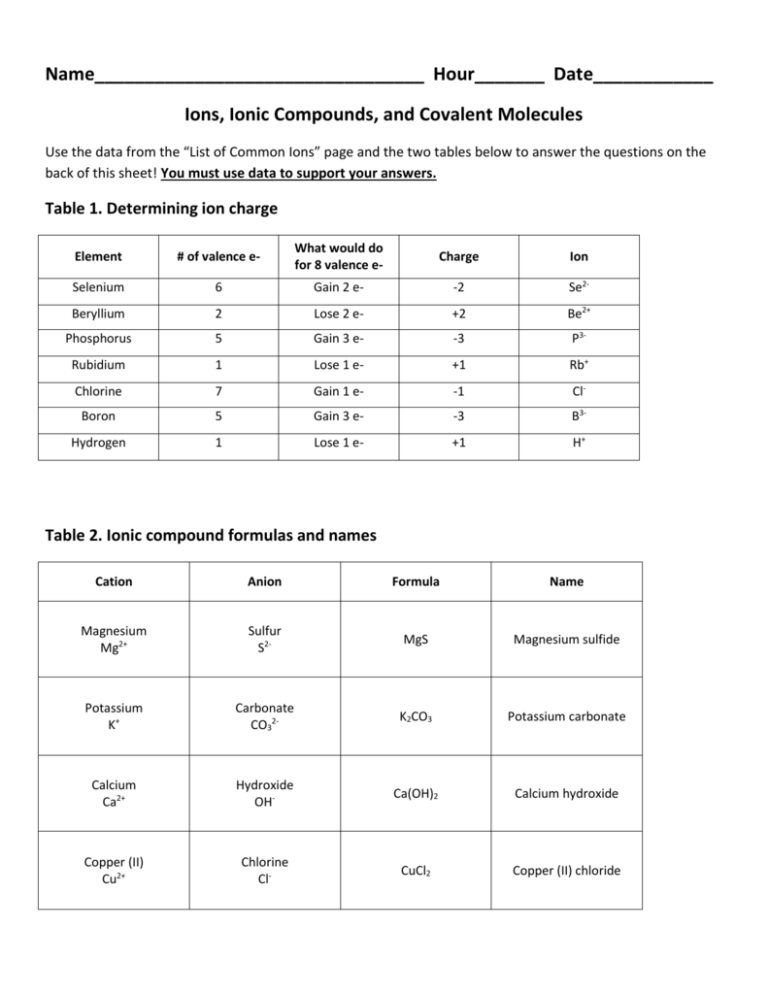
Determine the charge on the cation. Examples of names of ionic compounds. Therefore NaCl is named sodium chloride.
Using your periodic table identify the ions that are in each compound. A Mg Cl042 b NH4NO3 c Cu CH3CO22 d K2S03. Match each name of a charged or uncharged particle to its description.
So the oxidation number of Mn in MnO is 2 and the name of the compound is manganeseII oxide. Rubidium lose 1 cation f. Give names for the ionic compounds below.
Give names for the ANION in the ionic compounds below. It is written SrCl 2. Iron II iron III gallium.
Naming an ionic compound. The oxidation number of Mn in MnO₂ is 4 and the name of the compound is manganeseIV oxide. Heres a video on naming and writing formulas for ionic compounds with Roman.
Select the properly written formulas for ionic compounds from the list below. The most basic ionic compounds use a metal as the cation and a non-metal as the anion and just has the two elements. Strontium lose 2 cation c.
Give names for the ionic compounds below. The most common ions with multiple oxidation numbers are shown below. Monoatomic Anions take the elements name and ends with -ide ex.
- Name the anion. For example sodium chloride consists of Na1 and Cl-1. If needed use the systematic names.
Below are some examples of binary ionic compounds. Write chemical formulas for the compounds in each box. Correctly order the steps used in naming an ionic compound with a given formula.
Using the IUPAC nomenclature rules and the periodic table choose the correct name for the following ionic compounds. This includes compounds such as. A chlorine atom gains one electron.
Lithium oxide _____ Magnesium chloride _____.

Ions Ionic Bonds 3 2 1 Cie Igcse Chemistry Revision Notes 2020 Save My Exams
What Are Three Facts About Ionic Bonds Quora
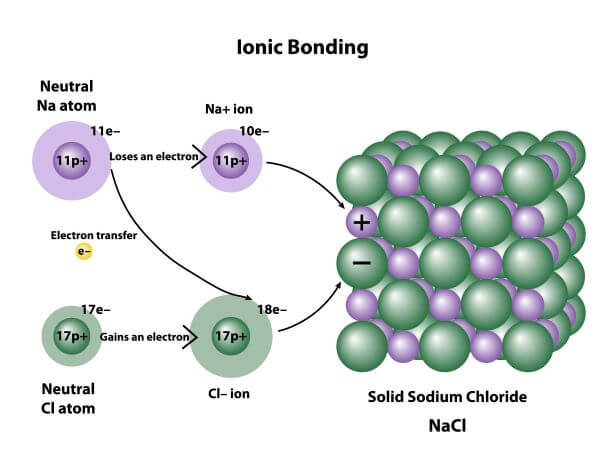
Ionic Bond Examples Biology Dictionary

Rules For Naming Ionic Compounds Video Lesson Transcript Study Com

Anion Common Anions Their Names Formulas And The Elements They Are Derived From Physical Science Names Physics

Ionic Bond Examples Biology Dictionary
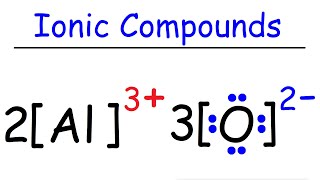
How To Draw The Lewis Structures Of Ionic Compounds Youtube
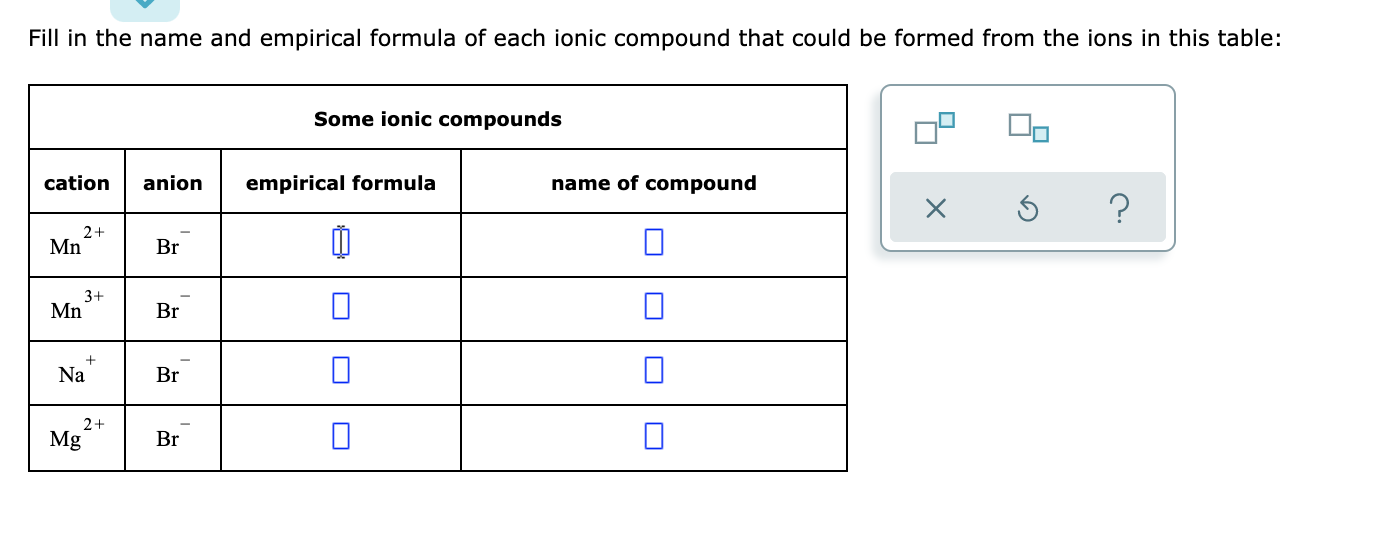
Solved Fill In The Name And Empirical Formula Of Each Ionic Chegg Com
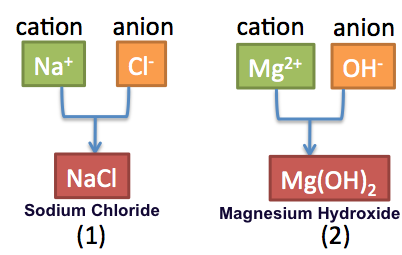
Rules For Naming Ionic Compounds Video Lesson Transcript Study Com
(187).jpg)
Naming Ionic Compounds Quiz Proprofs Quiz

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
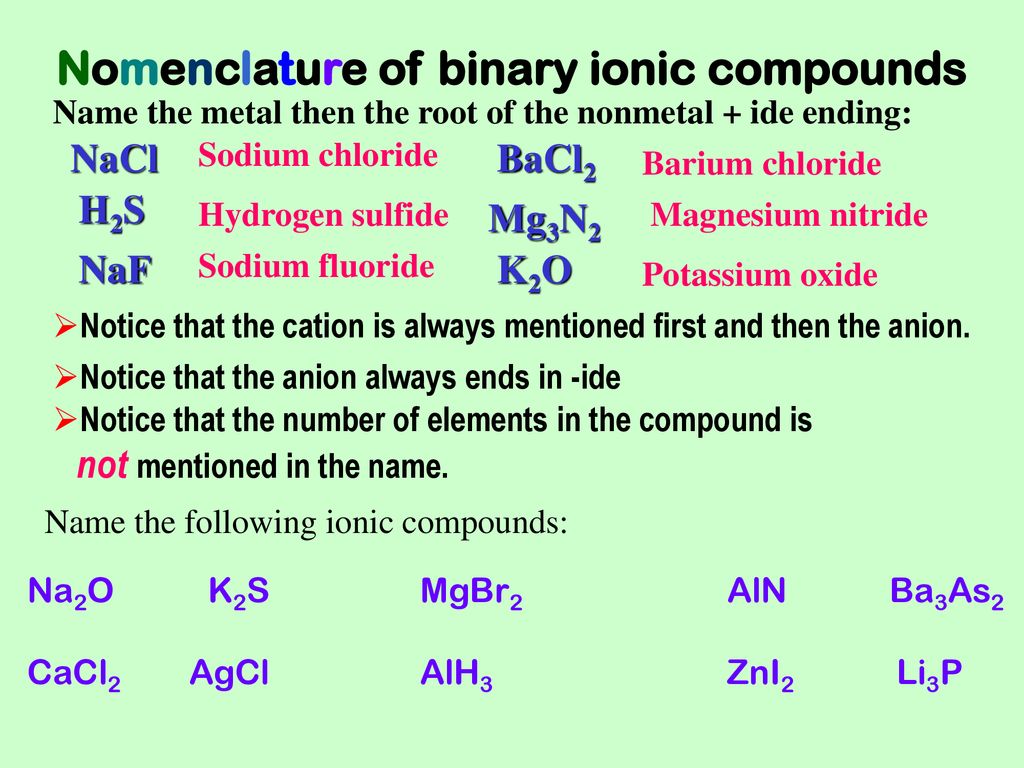
Naming Ionic Compounds Ppt Download
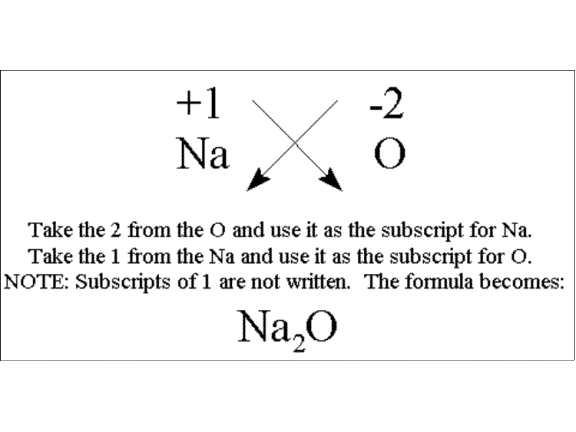

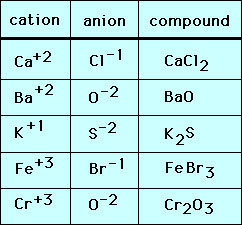
Comments
Post a Comment